The CORE-MD project has been launched in close collaboration between the European Federation of National Associations of Orthopaedics and Traumatology and the European Society of Cardiology. It is part of the European Union’s Horizon 2020 research and innovation programme. Through its research, the CORE-MD team aims to review methods for assessing high-risk medical devices, including artificial intelligence algorithms.
A project to monitor the quality of medical devices
The launch of this project comes in the context of the entry into force on 26 May of new European regulations on medical devices, in particular Regulation (EU) 2017/745, which strengthens the requirements for clinical evidence for high-risk medical devices.
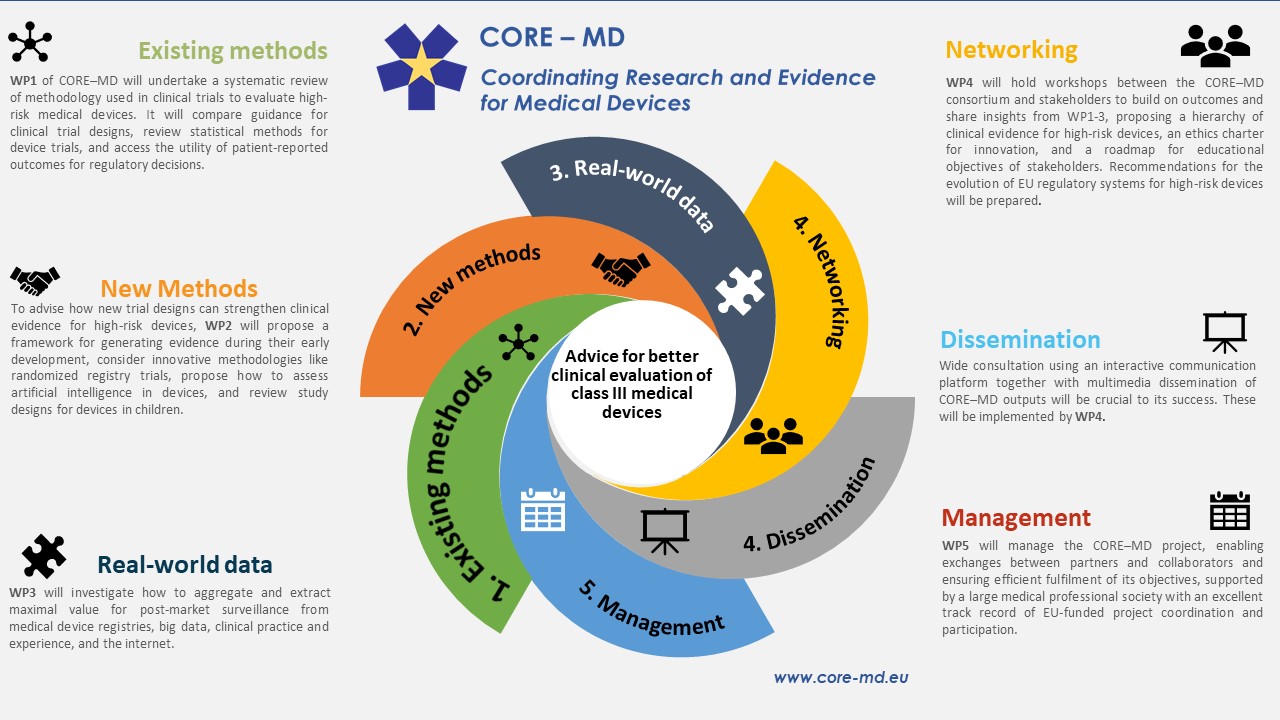
CORE-MD (Coordinating Research and Evidence for Medical Devices) is a formalised group of stakeholders in Europe comprising twenty-two partners working together to identify ways in which medical devices can be scientifically, systematically and fairly assessed. The project will last three years and will focus on heart valves and hip replacements which constitute more than 50% of the high-risk implantable medical devices in Europe.
Evaluation of algorithms and innovative methodologies
CORE-MD is structured around three main axes:
- A systematic review of the methodologies used in clinical trials to evaluate high-risk medical devices.
- How do we evaluate medical devices used in children?
- How to generate evidence using innovative methodologies such as randomized registry trials, and how to evaluate artificial intelligence algorithms that are considered medical devices?
The answer to this last point could constitute a real breakthrough in the medical device analysis sector.
The project is also divided into 5 “work packages”:
- Work package 1 (WP1) will focus on understanding the methods that have been used to generate clinical evidence for high risk medical devices.
- Work Package 2 (WP2) aims to strengthen the clinical evidence for high-risk medical devices by exploring new methods to generate data on their performance.
- Work Package 3 (WP3) aims to extract maximum value from medical device registries and real world evidence.
- Work packages 4 and 5 (WP4 and WP5) will focus on stakeholder engagement and project management respectively, these are the logistics work packages.
The project aims to improve the benefit/risk ratio of new medical devices. Within three years, the CORE-MD recommendations will be submitted to the European Commission’s Working Party on Clinical Research and Evaluation so that it can use this guidance to develop common EU guidance documents or specifications.
Translated from Focus sur le projet CORE-MD qui propose une revue des méthodes d’évaluation des dispositifs médicaux